Research
Control & Characterization of Peptide Self-Assembly Process
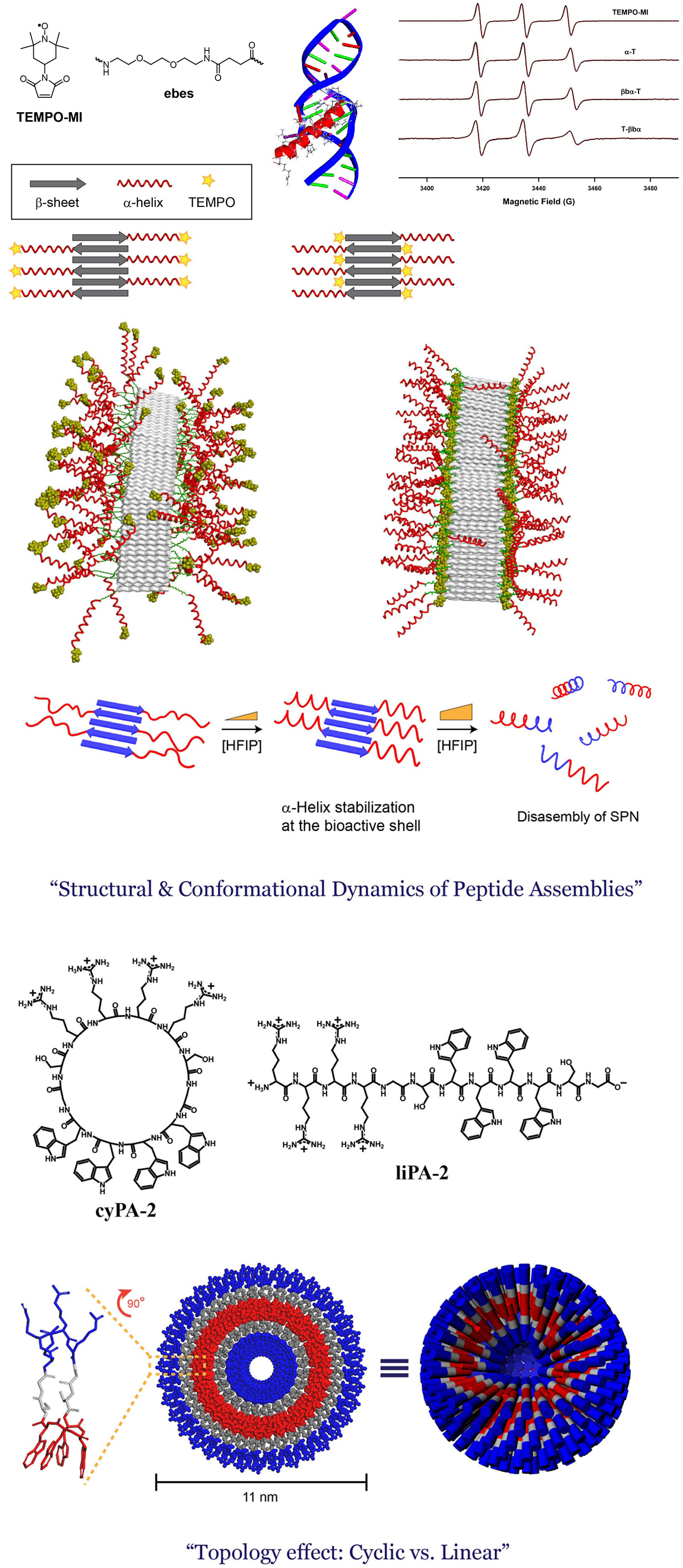
Self-assembled peptide nanostructures (SPNs) have great potential as promising biomaterials. Unlike proteins that consist of very long polypeptide chains with hundreds or more amino acids, SPNs are composed of peptides, i.e., small fragments of proteins typically with less than 50 amino acids in size. Typically, hundreds or more than thousands of peptide building block molecules aggregate to form SPNs, resulting in the formation of high molecular weight aggregates. Therefore, despite their marked difference in their building block size (peptides vs. very long polypeptides in natural proteins), the sizes of SPNs are comparable to or occasionally larger than those of proteins due to the process of bottom-up self-assembly. Researches, even though still significantly primitive when compared to natural proteins, are in progress to devise SPNs that can mimic or displace the diverse biological functions of natural proteins, possibly with enhanced properties or with functions that are unprecedented in nature. Understanding the self-assembly behavior of peptides is important during the development of controllable and tailor-made nanomaterials. This is particularly true for nanostructures that are intended for biological applications because biomolecules are usually highly sophisticated matters. We are trying to understand the self-assembly process of peptide-based supramolecular building blocks using a variety of nanostructural and biophysical techniques, which is a starting point for the fabrication of controllable and tailor-made SPNs.