Research
Modulation of Cellular Interactions using Self-Assembled Peptide Nanostructures
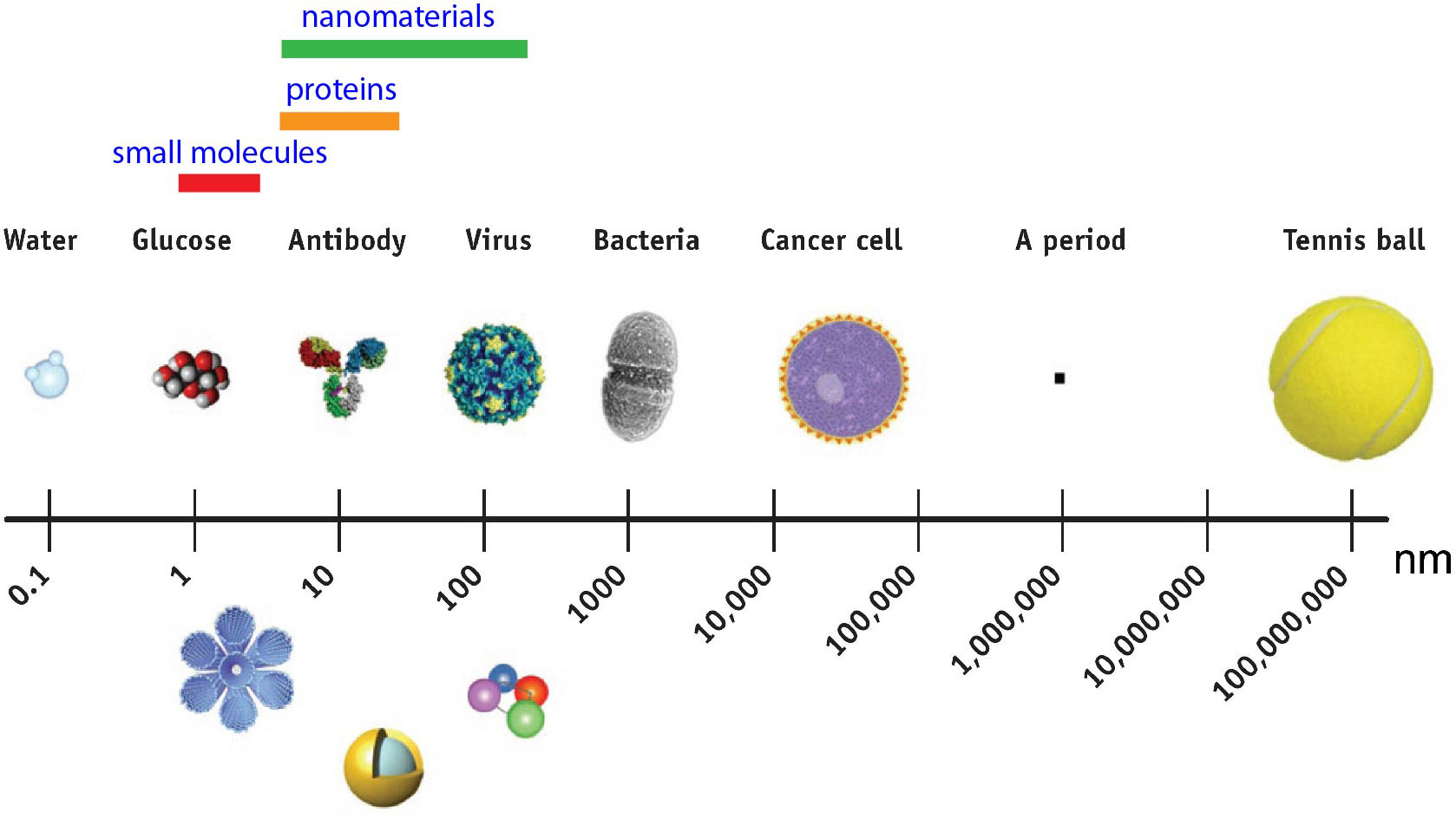
Nanostructures, or nanoparticles, have been successfully developed as nanomedicines for the treatment of many diseases. The materials that are used to fabricate nanomedicines are diverse and include polymers, lipids, peptides, inorganic nanoparticles, carbon nanotubes, and conjugates or hybrids of these materials. The majority of nanomedicines that have been developed to date do not exhibit therapeutic effects by themselves. Instead, these medicines have been used primarily as carriers to deliver substances that have therapeutic effects such as drugs, genes, peptides, and proteins. To go beyond the role of nanomedicines just as passive delivery carriers, it is necessary to develop more sophisticated nanomedicines that have novel active biological functions. We aim to develop a nanomedicine that is pharmaceutically active by itself. From the stand point of size, nano-sized objects are similar to many subcellular components (e.g., proteins and DNA) and cellular organelles; therefore, when nanostructures are appropriately designed and fabricated, a huge potential exists for these materials to control myriad interactions that are mediated by specific molecular recognition events between nano-sized biological structures. Because biomacromolecular interactions (BMI, e.g., protein-protein, protein-DNA, and protein-RNA interactions) are the most essential biomacromolecular interactions, we are pioneering to create nanostructures that can modulate these types of interactions.
BMIs are involved in cellular communication and a number of disease pathways, and take place between relatively large biological entities. This size issue, when combined with the typically wide and shallow nature of many biomacromolecular interfaces, makes the development of BMI inhibitors using conventional small molecules very difficult. Larger biomacromolecules such as therapeutic antibodies and peptides have shown some success in modulating BMIs, but the associated problems such as the thermal/structural instability, high susceptibility to proteolysis, difficulty in manufacturing, and high costs for the production of these molecules still exist.
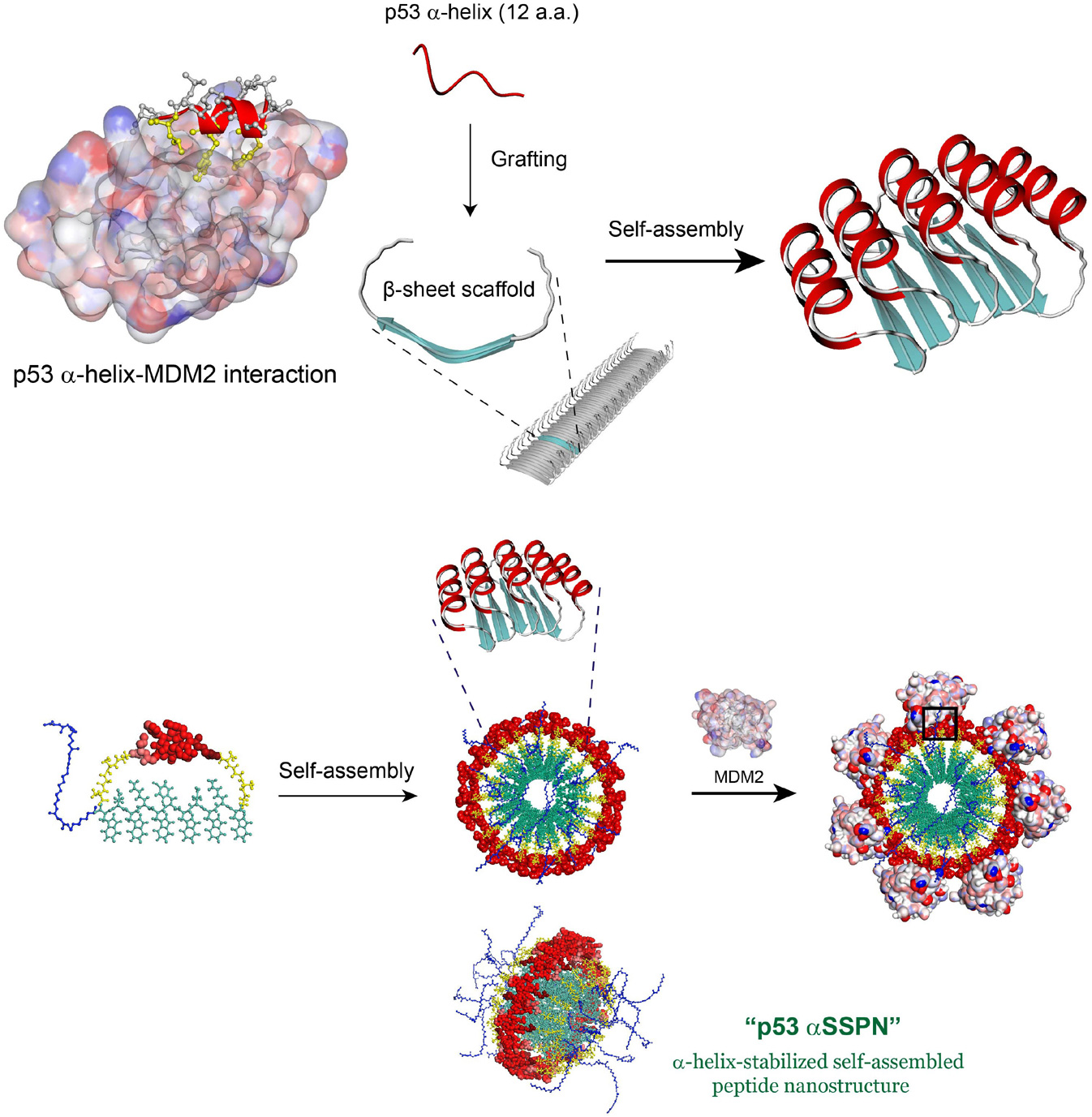
a-Helices comprise more than 30% of the secondary structures that are found in globular proteins. Importantly, many a-helices that are localized at the protein surface are involved in protein-protein, protein-DNA, and protein-RNA interactions. Although a-helical structures are well stabilized in the context of intact proteins, they tend to be unstructured when isolated from the protein as monomeric peptides because of their inherent thermodynamic instability. To circumvent this problem, many attempts, such as stapled peptide approaches, have been made to stabilize a-helical structures in the framework of peptides, which is critical for achieving high affinity and specificity. However, these methods generate a stabilized a-helical peptide that is only present in a monomeric form and often includes deleterious chemical modifications of the peptide.
Our approach is rooted in the bottom-up self-assembly method. Self-assembled peptide nanostructures (SPNs) containing multiple intact stabilized a-helical structures at the nanostructural surface can be constructed by the self-assembly of macrocyclic peptides. By grafting an a-helical peptide from the active site of natural proteins into the self-assembling macrocyclic peptide scaffold, we intend to develop a-helix-stabilized multivalent SPNs that can modulate a-helix-mediated BMIs.